Lipitor
Treating Patients With Diabetes
LIPITOR® Provides Significant CV Risk Reduction in Patients with Diabetes1
Hypertension + Diabetes + ≥2 Additional Risk Factors
In an ASCOT-LLA post hoc subgroup analysis in patients with hypertension, diabetes, and ≥2 additional risk factors, LIPITOR® 10 mg reduced the risk of total CV events and procedures by 23% compared with placebo2

Adapted from Sever PS et al. Diabetes Care. 2005.
ASCOT-LLA Study Design2
- Double-blind, placebo-controlled study including 10,305 hypertensive patients treated with antihypertensive therapy and without a previous MI with TC ≤250 mg/dL (6.5 mmol/L)
- Patients also had ≥3 additional CV risk factors
- Randomized to LIPITOR® 10 mg (n=5168) or placebo (n=5137)
- Trial was terminated early (after a median follow-up of 3.3 years) on the recommendation of the data safety monitoring board, due to the significant early difference between groups in the primary endpoint.
- Analysis includes the 2532 patients with diabetes randomized to LIPITOR® (n=1258) or placebo (n=1274)
Diabetes + ≥1 Additional Risk Factor
In the CARDS trial in patients with type 2 diabetes and ≥1 other CHD risk factor, LIPITOR® 10 mg reduced the risk of major CV events by 37% compared with placebo1

*Major CV events: MI (including silent MI), unstable angina, acute CHD death, resuscitated cardiac arrest, coronary revascularization, and stroke.4
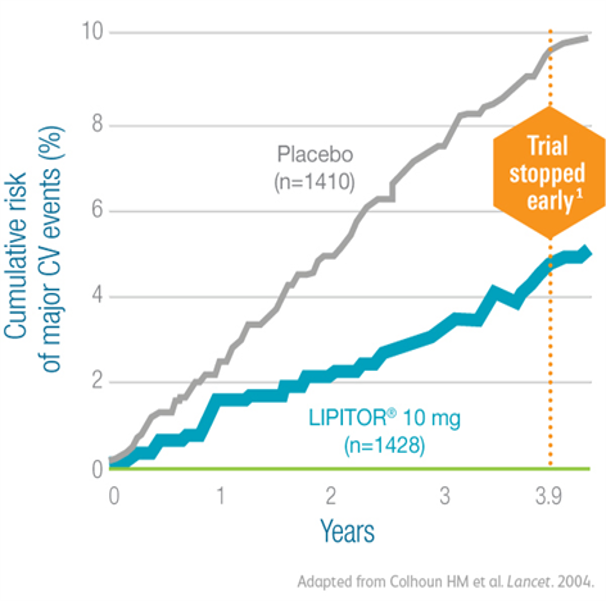
- LIPITOR® 10 mg also reduced the risk of MI by 42% (P=0.007)3
In the CARDS trial in patients with type 2 diabetes and ≥1 risk factor, LIPITOR® 10 mg reduced the risk of stroke by nearly half compared with placebo4

*Major CV events: MI (including silent MI), unstable angina, acute CHD death, resuscitated cardiac arrest, coronary revascularization, and stroke.4
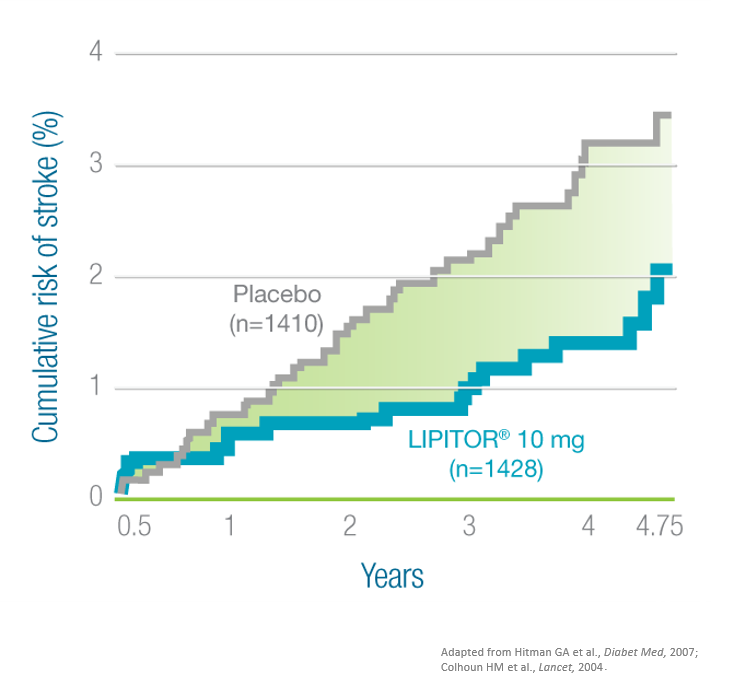
- The lowest mean LDL-C level reached with LIPITOR® in this study was 68 mg/dL (1.8 mmol/L)1
CARDS Study Design1,4,5
- Double-blind, prospective study in which 2838 patients aged 40 to 75 years were randomized to LIPITOR® 10 mg (n=1428) or placebo (n=1410)
- Patients had type 2 diabetes, no documented history of CVD, LDL-C ≤160 mg/dL, TG ≤600 mg/dL, and ≥1 of the following: hypertension, retinopathy, albuminuria, or current smoking
- The primary endpoint was time to first occurrence of acute CHD events, coronary revascularization, or stroke
- Median duration of follow-up was 4.0 years (IQR 3.0-4.7) in the atorvastatin group; the CARDS trial was terminated at 3.9 years—2 years earlier than planned—due to highly significant benefits of atorvastatin
Diabetes + CKD,
In the CARDS post hoc subgroup analysis in high-risk patients with type 2 diabetes and CKD*, LIPITOR® 10 mg significantly reduced the risk of stroke compared with placebo5

- In the CARDS post hoc subgroup analysis, LIPITOR® 10 mg (n=482) reduced mean LDL-C from baseline 120 mg/dL to 71 mg/dL5
CARDS Study design1,4,5
- Double-blind, prospective study in which 2838 patients aged 40 to 75 years were randomized to LIPITOR® 10 mg (n=1428) or placebo (n=1410)
- Patients had type 2 diabetes, no documented history of CVD, LDL-C ≤160 mg/dL, TG ≤600 mg/dL, and ≥1 of the following: hypertension, retinopathy, albuminuria, or current smoking
- The primary endpoint was time to first occurrence of acute CHD events, coronary revascularization, or stroke
- Median duration of follow-up was 4.0 years (IQR 3.0-4.7) in the atorvastatin group; the CARDS trial was terminated at 3.9 years—2 years earlier than planned—due to highly significant benefits of atorvastatin
ASCOT-LLA: Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm; CARDS: Collaborative AtoRvastatin Diabetes Study; CHD: coronary heart disease; CV: cardiovascular; CVD: cardiovascular disease; CKD: chronic kidney disease; HDL-C: high-density lipoprotein cholesterol; IQR: interquartile range; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; RRR: relative risk reduction; TC: total cholesterol; TG: triglycerides.
References:
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696.
- Sever PS, Poulter NR, Dahlöf B, et al; ASCOT Investigators. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA). Diabetes Care. 2005;28(5):1151-1157.
- LIPITOR® (atorvastatin calcium) Summary of Product Characteristics. January 2019 for UAE, Qatar, Iraq, Bahrain, Oman and June 2016 for Kuwait.
- Hitman GA, Colhoun H, Newman C, et al. Stroke prediction and stroke prevention with atorvastatin in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabet Med. 2007;24(12):1313-1321.
- Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kid Dis. 2009;54(5):810-819.
LIPI-2024-0062